Design of Diradical Generator Agents for Biological Substrates
Nature has created a vast array of remarkable and structurally complex natural products to perform unusual and biochemically relevant functions. There are two broad types of these natural products; those that perform function via binding inhibition or activation, and those that are chemically very reactive and potent to biological targets. The former category gives way to lead organic compounds for the drug industry, while the latter are often disregarded as too toxic. There also exists a smaller array of inorganic therapeutic agents that take advantage of the much more diverse reactivity that metals can offer (Cisplatin, MRI contrast agents, nuclear medicinal agents,etc). Interestingly, like the reactive natural products, these agents all perform some type of reaction chemistry, but the reason they have made it to utility is that research has found ways to control this biological reactivity.
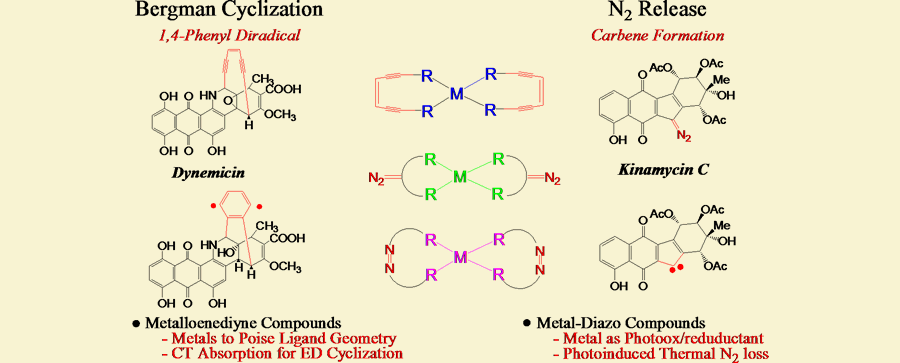
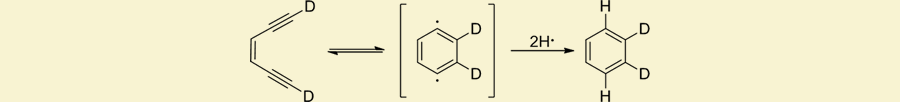
One of our major research efforts is to develop inorganic medicinal agents based on the lessons we have learned from highly reactive natural products. We have chosen two natural product families as molecular templates for our inorganic designs; the enediyne and the kinamycin families of agents. The former contains the biosynthetically startling (Z)-1,5-diyne-3-ene unit that upon redox activation, rearranges to a 1,4-didehydrobenzene diradical intermediate and performs H-atom abstraction from the phosphodiester backbone, cleaving the DNA and inducing apoptosis. In the kinamycin family, similar redox activation promotes extrusion of N2 and carbenediradical formation leading to either DNA adducts or H-atom abstraction and strand scission.
Our research goals are to control the chemistry of these highly reactive functional groups through the use of the geometric and electronic properties of metal ions which are chemically more diverse than carbon. In the case of enediynes, small molecule frameworks are synthesized that geometrically control the interalkynyl distance, thereby stabilizing or activating the enediyne for diradical formation. Similar effects can be achieved electronically by controlling the oxidation state of the metal or the substituents near the alkyne termini to withdraw electron density from the alkyne in-plane π-system.
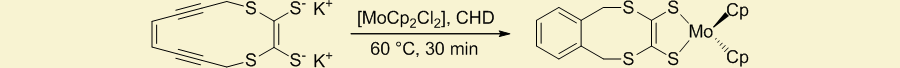
Additionally, we have shown that coordination to biologically prevalent, non-toxic metals can be used to promote cycloaromatization of the enediyne moiety at ambient temperatures.
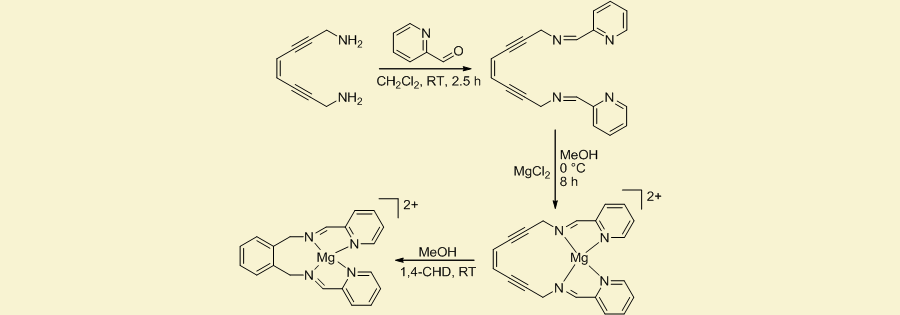
Current work in our laboratory focuses on the development of enediyne-containing anti-tumor therapeutics based on Cisplatin using Pt2+ to employ a square-planar geometry in an effort to control the cyclization temperature. Additionally, we are investigating the use of ligands for biologically-inherent metal centers such as Cu2+ and Fe2+, two metals known to play a role in the aggregation of plaques in Alzheimer�s disease, for in situ chelation to induce cyclization and subsequent radical generation. In addition to thermal activation, we are also exploring the use of metal ion redox pairs to instantaneously promote geometry changes in order to induce the cyclization event and diradical formation.

