Plasmonic Nanomaterials for Antibiotic Resistance, Clots, and Biofilms
It is projected that antibiotic resistance (AR) could kill an estimated 10 million people annually by the year 2050. Understanding the AR burden through world-wide trends and drug-pathogen contributions are critical to gaining a better estimate of potential future lethality that requires immediate interventions to ward off. There are three primary contributors to AR, two of which involve impeding antibiotic concentration buildup inside bacteria through upregulation of efflux pumps or diminished outer membrane (OM) permeability. The third depends upon antibiotic modification, where for example, enzymes such as b-lactamases ring-open the 4-membered lactam ring of penicillin-inspired antibiotics.
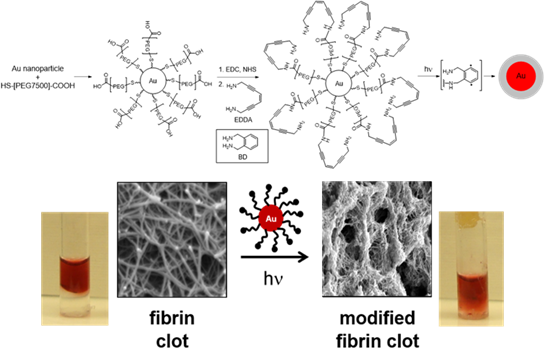 Figure 1. Au nanospheres with pegylated
Figure 1. Au nanospheres with pegylated
diradical generating EDDA-enediyne coating degrade
fibrin clots and restore flow in
6 h at 514 nm (100 mW).
In an effort to attack biological substrates in general, we have synthesized an array of nanomaterials for a range of biological and catalysis applications by focusing on taking advantage of the properties of the particle itself, or the designed surface decoration to realize the desired outcome. A considerable amount of this effort has concentrated on the ability to substitute nanomaterial surfactants with customized surface ligands that can be activated to form a potent 1,4-phenyl diradical species reminiscent of the enediyne family of antibiotics that perform H-atom abstraction from biological substrates (e.g. DNA). In non-aqueous media, photolysis of Au nanoparticles at the SPR band (~520 nm, 100 mW, 1 hr; Figure 1), results in rapid diradical formation near the particle surface via non-radiative decay of the excitation to heat. In the absence of substrate, the diradicals will add across the alkyne of an adjacent molecule or radical quench each other to form carbon-carbon bonds around the surface leading to visible polymer coatings in the TEM. This effect can be reproduced thermally over 8 hr at 50 °C, verifying the process as photothermal in origin. The model is also operative for Au nanorods whose LSPR is at 785 nm, resulting in polymer coated rods upon photolysis within 12 hr.
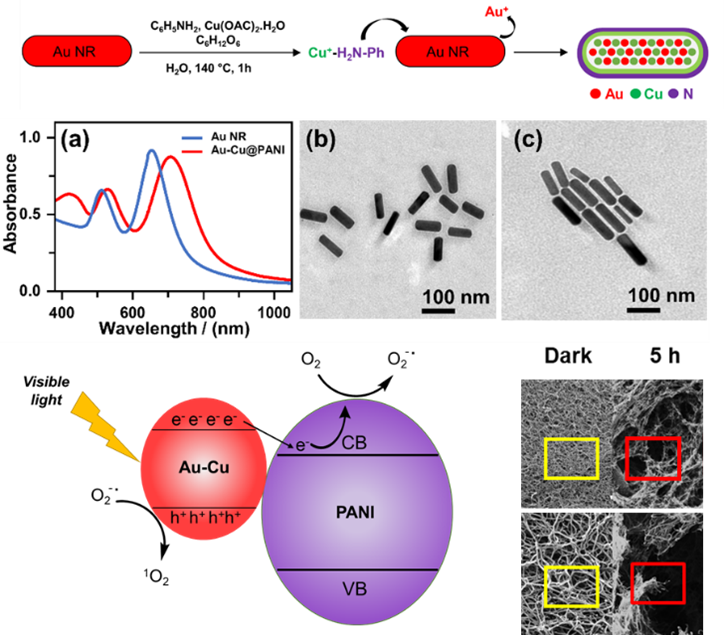 Figure 2. Electron-rich, hybrid n-doped Au-Cu
Figure 2. Electron-rich, hybrid n-doped Au-Cu
rods (a-c) demonstrating photo-redox
formation of ROS that leads to
fibrin clot degradation in 5 h.
Furthermore, we have used this paradigm to create reactive 1O2 via photoinduced superoxide O2− formation upon photoexcitation of Au-CuPANI hybrid nanorods at 785 nm (Figure 2). Mechanistically, the ROS can be readily detected by dye bleaching and EPR spin trapping, indicating that the photoredox reaction occurs through superoxide. When applied to crosslinked fibrin clots, similar outcomes were observed relative to our earlier enediyne degradation results. Clots showed marked in increase in fiber ends indicating substantial scission of fibers as well as the appearance of network collapse. Both indicators suggest that nanomaterial-generated ROS, or even ROS formed biologically, can show non-enzymatic biological substrate degradation. One under investigated challenge is quantitatively comparing these outcomes, while a second relates to how a living biological system would react to such a threat. Do organisms develop resistance to ROS? If so, what does that genetic pathway look like. Could diradical generation supersede that evolutionary response, and if not free in solution, could concentrated multi-radical damage delivered by a nanomaterial result in a more difficult invader for organisms to develop resistance to. At minimum, understanding the response organisms mount to CMRD or its ROS counterpart will provide a window into genetic responses of organisms and better define approaches to combat the growing global threat of antibiotic resistance.
The nanomaterial constructs utilize the optical or magnetic properties of the platform nanomaterial to induce thermally activated, multi-radical generation from the enediyne surfactant at the site of target localization. Since the diradical generating nanomaterial activation strategy is capable of concentrated, multi-radical damage at 20-70 nm site on biological substrates, we have a unique opportunity to evaluate how bacteria react to this high radical density.
Related Publications
- Au-Cu@PANI Alloy Core Shells for Aerobic Fibrin Degradation under Visible Light Exposure, Riyadh H. Alshammari, Ummadisetti Chinna Rajesh, David Gene Morgan, and Jeffrey M. Zaleski; ACS Appl. Bio. Mater. (2020), 3, 7631-7638.
- The Outliers: Inorganic Radical Reagents for Biological Substrate Degradation, Meghan R. Porter, Joan M. Walker, Jeffrey M. Zaleski, Accts. Chem. Res., (2019), 52(7) 1957-1967.
- Electrostatic Assembly of Gold Nanorods on a Glass Substrate for Sustainable Photocatalytic Reduction via Sodium Borohydride, Jordan Harrington, Jeffrey M. Zaleski, RSC Advances (2016), 6, 59113-59123.
- Magnetically-Triggered Radical-Generating Fe3O4 Nanoparticles for Biopolymer Restructuring: Application to the Extracellular Matrix, Joan M. Walker, Jeffrey M. Zaleski, Chem. Mater., (2015), 27 (24), 8448–8456.
- Non-Enzymatic Remodeling of Fibrin Biopolymers via Photothermally-Triggered Radical-Generating Nanoparticles, Joan M. Walker, Jeffrey M. Zaleski, Chem. Mater., (2014) 26 (17), 5120–5130.
- Expansion and Contraction: Shaping the Porphyrin Boundary via Diradical Reactivity, Leigh J.K. Boerner, David F Dye, Tillmann Köpke, Jeffrey M. Zaleski, Chem. Rev., (2013), 257(2), 599-620.
- Convenient, Rapid Synthesis of Ag Nanowires, Linfeng Gou, Mircea Chipara, Jeffrey M. Zaleski, Chem. Mater., (2007), 19(7), 1755-1760.